Biocrystallographic and electrochemical studies of redox proteins
The respiratory system contains a series of electron carriers (complexes), most of which are integral proteins of the inner membrane, containing prosthetic groups able to accept and donate one or two electrons. To investigate the mechanisms of formation of macromolecular complexes and their dissociation after the electron transfer process, structural studies of iron (III) - and iron (II) - cytochrome c from horse heart are conducted, using nitrate as a probe anion to map the redox changes which dependent on the electrostatic surfaces. To obtain information on the mechanism of electron transfer which occurs between heme groups, the crystal structures of "cytochromes c dieme" proteins are studied.
In this context, electrochemical studies of electron transport proteins are also conducted. Cytochrome c molecules, of both horse heart and yeast (ycc), are adsorbed as self- assembled monolayers ( SAMs ) on polycrystalline Au electrode and studied using electrochemical techniques, in order to determine the thermodynamic parameters of the electronic transfer Fe (III) / Fe (II) under different experimental conditions (temperature, pH of the buffer, denaturing agents , the nature of the SAM ) .
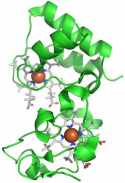 | 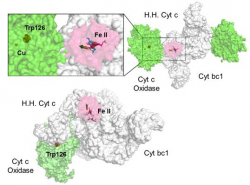 | 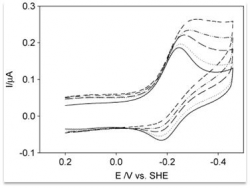 |
| High resolution crystal structure of the recombinant diheme cytochrome c from Shewanella baltica J. Biomol. Struct. Dyn. | Electron transfer complexes: the experimental mapping of the redox-dependent cytochrome c electrostatic surface supports the existence of the functional mitochondrial supercomplex iii-iv (respirosome) J. Inorg. Biochem. | Cyclic voltammetry for the unfolded His-His form of yeast cyt c adsorbed on a polycrystalline Au electrode coated with some self-assembled monolayer at increasing partial pressure of O2. Chem. Commun., 2011, 47, 11122 |
Research Group
| Redox proteins Group |