Heterochiral peptide self-assembly and nanomaterials
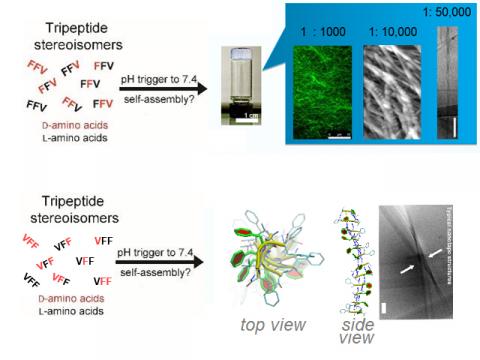
Short peptides (especially di- and tripeptides) are attractive building blocks for nanostructures and supramolecular materials, due to their simplicity and ease of preparation.1,2 Besides, tripeptides are also minimalist motifs capable of delivering a biological message to cells (e.g., RGD for cell adhesion).3
There are several approaches to drive self-assembly of short peptides. In particular, a relatively unexplored avenue is the use of chirality (i.e., appropriate choice of D- versus L-amino acids) to fine-tune the supramolecular behaviour of tripeptides and their ordered self-assembly into nanostructured materials. Recently, a few examples of hydrophobic tripeptides bearing D-amino acid(s) at selected positions have been reported to rapidly form nanostructured hydrogels under physiological conditions at which their homochiral stereoisomers (e.g., L-tripeptides) do not.4-7 This unexpected behaviour raises interesting questions that go beyond peptide self-assembly, including the transfer of chirality from simple molecules to supramolecular nanoarchitectures, and how these interact with life. Current investigations tackle:
- de novo design of self-assembling short peptides
- transfer of chirality from heterochiral tripeptides to supramolecular architectures
- preparation of hybrid or nanocomposite materials bearing different chemical components (e.g., peptides, carbon nanomaterials, inorganic nanoparticles, drugs, etc.) for added properties
- design of smart functional nanostructured materials (e.g., that respond to light, magnetic guidance, electric fields, etc.) for biological use (e.g., cell culture, tissue engineering, drug delivery)
References:
1. Nat. Chem. 2015, 7, 30.
2. Mol. Biosci. 2011, 11, 160.
3. J. Med. Chem. 2011, 54, 1111.
4. Chem. Commun. 2012, 48, 2195.
5. Nanoscale 2012, 4, 6752.
6. Nanoscale 2014, 6, 5172.
7. J. Mater. Chem. B 2015, 3, 8123.
Research Group
| Marchesan Group |