Development of metal complexes for imaging and therapy
The aim of this research line is that of developing metal compounds for multimodal diagnostic imaging and theranostic agents, i.e. compounds that combine in a single molecule the modalities of imaging and therapy. In particular, we aim to prepare Ru and Re precursors suitable for being conjugated to photodynamic therapy (PDT) agents, i.e. photosensitizers that – when irradiated with visible light – generate reactive oxygen species.
For example, a Ru-porphyrin conjugate might combine the phototoxicity and the tumour-localization properties of the porphyrin with the cytotoxicity of the metal fragment for additive antitumour effects, and the emission of the chromophore can be exploited for tracking the biodistribution of the adduct at the cellular level through fluorescence microscopy.
Conjugation with Re/99mTc congeners would provide a matched pair of compounds: the non-radioactive (cold) Re conjugate for chemical investigation, fluorescence imaging and PDT investigation, and the gamma-emitting (hot) 99mTc conjugate potentially suitable for bimodal molecular imaging (fluorescence and SPECT). A schematic example of the matched pair approach is drawn below. A recent publication on this topic is "Towards matched pairs of porphyrin-Re(I)/99mTc(I) conjugates that combine photodynamic activity with fluorescence- and radio-imaging." ChemMedChem 2014, 9, 1231–1237.DOI: 10.1002/cmdc.201300501.
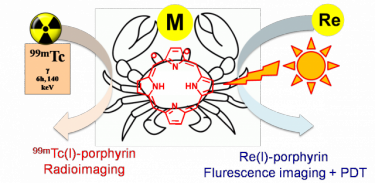
Thus, more specifically, we will develop Ru and Re precursors suitable for making conjugates with porphyrins that bear peripheral binding sites (from mono- to tridentate), that is complexes that have an appropriate number of coordination positions available for conjugation and ancillary ligands that are highly hydrophilic for improving solubility in water.
The most well known accomplishment in my career in the context of Medicinal Inorganic Chemistry was the development of NAMI-A, an antimetastatic Ru(III) coordination complex that is well known in the community of bioinorganic chemists (it even made its way to textbooks of Inorganic Chemistry, such as the 5th edition of the famous Shriver & Atkins). The formula of NAMI-A and an image of a macro-sample of the compound are shown below.
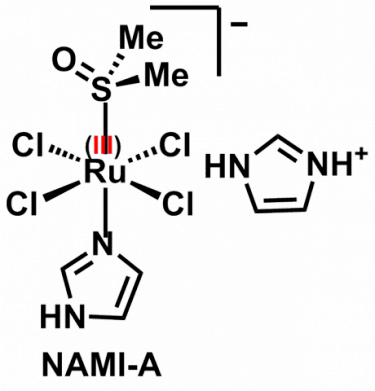
The pioneering role of my group – together with very few others – in the field of ruthenium anticancer drugs is well illustrated by the graph that reports the number of publications on this topic from 1990 to 2011.
When I started back in the late ‘80s there were basically two or three groups in the world working on this topic. At that time the focus was almost exclusively on Pt anticancer compounds. In 1999, by virtue of its excellent antimetsatic activity on model tumors in mice, NAMI-A was the first Ru complex ever to be tested on humans in a phase 1 clinical investigation. Since then, the number of publications on Ru anticancer complexes has been growing almost exponentially. From 2008 to 2011 NAMI-A, in combination with gemcitabine, was administered to 32 patients bearing non-small cell lung carcinoma in a phase 1-2 clinical investigation carried out at the Netherland Cancer Institute of Amsterdam. The results of the clinical study are currently being published:"Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy." Invest. New Drugs .... DOI: 10.1007/s10637-014-0179-1.
One of the main and distinctive characteristics of NAMI-A is its negligible toxicity in vitro against cell lines of solid tumors. A very recent, and totally unexpected finding, is that NAMI-A is highly cytotoxic toward leukaemia cell lines (see Dalton Trans. 2014, 43, 12150 – 12155. DOI: 10.1039/C4DT01356E.)
My role in the field of medicinal inorganic chemistry is well recognized in the scientific community of bioinorganic chemists. I have actively participated to all COST Actions in this field (since 1997, in COST Chemistry Action D1) and I served as vice-Chairman (2000-2003) and then Chairman (2003-2006) of COST Chemistry Action D20 ‘Metal compounds in the treatment of cancer and viral diseases’. In 2011 I was the editor for Wiley-VCH of the book entitled Bioinorganic Medicinal Chemistry (ISBN 978-3-527-32631-0) that puts together contributions of the most distinguished chemists in this field.
Pertinent publications from year 2009
1) T. Gianferrara, I. Bratsos, E. Alessio*
A Categorization of Metal Anticancer Compounds Based on Their Mode of Action.
Dalton Trans., 2009, 7588-7598. Perspective Article
2) T. Gianferrara, I. Bratsos, E. Iengo, B. Milani, A. Oštrić, C. Spagnul, E. Zangrando, E. Alessio*
Synthetic strategies towards ruthenium-porphyrin conjugates for anticancer activity.
Dalton Trans., 2009, 10742-10756.
3) T. Gianferrara, A. Bergamo, I. Bratsos, B. Milani, C. Spagnul, G. Sava, E. Alessio
Ruthenium-porphyrin conjugates with cytotoxic and phototoxic antitumor activity.
J. Med. Chem., 2010, 53, 4678-4690.
4) I. Bratsos, T. Gianferrara, E. Alessio,* C. G. Hartinger, M. A. Jakupec, B. K. Keppler
Ruthenium and Other Non-platinum Anticancer Compounds.
in Bioinorganic Medicinal Chemistry, E. Alessio ed., Wiley-VCH, Weinheim, 2011, pp. 151-174.
5) I. Bratsos,* D. Urankar, E. Zangrando, P. Genova-Kalou, J. Košmrlj, E. Alessio, I. Turel*
1-(2-picolyl)-substituted 1,2,3-triazole as novel chelating ligand for the preparation of ruthenium complexes with potential anticancer activity.
Dalton Trans. 2011, 40, 5188 – 5199.
6) I. Bratsos,* C. Simonin, E. Zangrando, T. Gianferrara, A. Bergamo, E. Alessio*
New half sandwich-type Ru(II) coordination compounds characterized by the fac-Ru(dmso-S)3 fragment: influence of the face-capping group on the chemical behavior and in vitro anticancer activity.
Dalton Trans. 2011, 40, 9533 – 9543.
7) I. Bratsos,* E. Mitri, F. Ravalico, E. Zangrando, T. Gianferrara, A. Bergamo, E. Alessio*
New half sandwich Ru(II) coordination compounds for anticancer activity
Dalton Trans. 2012, 41, 7358 – 7371.
8) A. Rilak, I. Bratsos,* E. Zangrando, J. Kljun,I. Turel, Ž. D. BugarÄić, E. Alessio*
Factors that influence the antiproliferative activity of half sandwich RuII-[9]aneS3coordination compounds: activation kinetics and interaction with guanine derivatives.
Dalton Trans. 2012, 41, 11608 – 11618.
9) G. Ragazzon, I. Bratsos, E. Alessio,* L. Salassa,*A. Habtemariam, R. McQuitty, G. J. Clarkson, P. J. Sadler*
Design of Photoactivatable Metallodrugs: Selective and Rapid Light-induced Ligand Dissociation from Half-Sandwich [Ru([9]aneS 3 )(N–N')(py)]2+ Complexes.
Inorg. Chim. Acta 2012, 393, 230-238.
10) C. Spagnul, R. Alberto,* G. Gasser, S. Ferrari, V. Pierroz, A. Bergamo, T. Gianferrara,* E. Alessio
Novel water-soluble 99mTc(I)/Re(I)-porphyrin conjugates as potential multimodal agents for molecular imaging.
J. Inorg. Biochem. 2013, 122, 57–65.
11) I. Finazzi, I. Bratsos, T. Gianferrara, A. Bergamo, N. Demitri, G. Balducci, E. Alessio*
Photolabile RuII Half-Sandwich Complexes Suitable for Developing “Caged†Compounds: Chemical Investigation and Unexpected Dinuclear Species with Bridging Diamine Ligands.
Eur. J. Inorg. Chem. 2013, 4743–4753. DOI:10.1002/ejic.201300792
12) J. Kljun,I. Bratsos, E. Alessio, G. Psomas, U. Repnik, M. Butinar, B. Turk, I. Turel*
New Uses for Old Drugs: Attempts to Convert Quinolone Antibacterials into Potential Anticancer Agents Containing Ruthenium.
Inorg. Chem. 2013, 52, 9039–9052. DOI: 10.1021/ic401220x
13) T. Gianferrara, C. Spagnul,R. Alberto,* G. Gasser,S. Ferrari, V. Pierroz, A. Bergamo, E. Alessio*
Towards matched pairs of porphyrin-Re(I)/99mTc(I) conjugates that combine photodynamic activity with fluorescence- and radio-imaging.
ChemMedChem 2014, 9, 1231–1237.DOI: 10.1002/cmdc.201300501.
14) A. Rilak, I. Bratsos,* E. Zangrando, J. Kljun, I. Turel, ZÌŒ. D. BugarcÌŒicÌ, E. Alessio*
New Water-Soluble Ruthenium(II) Terpyridine Complexes for Anticancer Activity: Synthesis, Characterization, Activation Kinetics, and Interaction with Guanine Derivatives.
Inorg. Chem. 2014, 53, 6113–6126. DOI 10.1021/ic5005215.
15) S. Pillozzi, L. Gasparoli, M. Stefanini, M. D’Amico, M. Ristori, E. Alessio, F. Scaletti, A. Becchetti, A. Arcangeli*, L. Messori*
NAMI-A is Highly Cytotoxic Toward Leukaemia Cell Lines: Evidence of Inhibition of KCa3.1 Channels.
Dalton Trans. 2014, 43, 12150 – 12155. DOI:10.1039/C4DT01356E.
Research Group
| Prof. Alessio's Group |