Solvent-free technological processes: melt-extrusion and spray congealing
This area of research consists in the production of oral solid dosage forms by means of a single technological step and in the absence of solvents. The main ingredients are pharmaceutical grade excipients with low melting point which are melted or softened during the process, and act as binder and / or carrier for the active ingredient. With appropriate choice of the material, the drug release can be appropriately tailored (performing an accelerated or retarded release).
In the case of the spray-congealing, microspheres or microcapsules, characterized by highly spherical shape and highly uniform size can be produced by spraying with ultrasounds the carrier. The drug can be included in a dissolved or dispersed state depending on the affinity with the excipient.
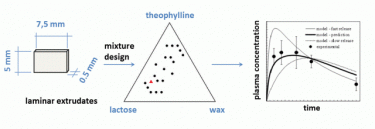
In the case of melt extrusion (and melt co-extrusion), it is possible to realize extrudates from the desired shape (solid and hollow cylinders, helices, sheet, etc.) through the use of dies of different shape. The selection of the process (temperature, pressure, etc.) and formulation variables (amount of softened binder, auxiliary fillers and active) permits together with the different mass-to-surface ratio of the different shapes to obtain the desired in vitro and in vivo drug release (as presented in the figure).
Collaborations:
Mario Grassi (Dept. Engineering and Architecture), Barbara Campisi (DEAMS), Nadia Passerini (Univ. of Bologna), Beatrice Albertini (Univ. of Bologna), Stefano Dall’Acqua (Univ. of Padova) Peter Kleinebudde (H. Heine University), João F. Pinto (Univ. Lisbon), Iztok Grabnar (Univ. Ljubljiana), Roger Phan-Tan-Lou (Univ. Aix-en- Provence).
Pubblications:
- Hasa, D., Perissutti, B., Campisi, B., Grassi, M., Grabnar, I., Golob, S., Mian, M., Voinovich, D. Quality improvement of melt extruded laminar systems using mixture design European Journal of Pharmaceutical Sciences. 75 (2015) 169-176.
- Passerini, N., Perissutti, B., Albertini, B., Franceschinis, E., Lenaz, D., Hasa, D., Locatelli, I., Voinovich, D. A new approach to enhance oral bioavailability of Silybum Marianum dry extract: Association of mechanochemical activation and spray congealing. Phytomedicine Volume 19, Issue 2, 15 January 2012, Pages 160-168.
- Hasa, D., Perissutti, B., Grassi, M., Zacchigna, M., Pagotto, M., Lenaz, D., Kleinebudde, P., Voinovich, D. Melt extruded helical waxy matrices as a new sustained drug delivery system. European Journal of Pharmaceutics and Biopharmaceutics. Volume 79, Issue 3, November 2011, Pages 592-600.
- Quintavalle, U., Voinovich, D., Perissutti, B., Serdoz, F., Grassi, G., Dal Col, A., Grassi, M. Preparation of sustained release co-extrudates by hot-melt extrusion and mathematical modelling of in vitro/in vivo drug release profiles. European Journal of Pharmaceutical Sciences. Volume 33, Issue 3, 3 March 2008, Pages 282-293.
- Quintavalle, U., Voinovich, D.,Perissutti, B., Serdoz, F., Grassi, M. Theoretical and experimental characterization of stearic acid-based sustained release devices obtained by hot melt co-extrusion Journal of Drug Delivery Science and Technology Volume 17, Issue 6, November 2007, Pages 415-420.
- Albertini, B. , Passerini, N., González-RodrÃguez, M.L., Perissutti, B., Rodriguez, L. Effect of Aerosil® on the properties of lipid controlled release microparticles. Journal of Controlled Release Volume 100, Issue 2, 24 November 2004, Pages 233-246.
- Passerini, N., Perissutti, B., Albertini, B., Voinovich, D., Moneghini, M., Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing Rodriguez, L. Journal of Controlled Release Volume 88, Issue 2, 7 March 2003, Pages 263-275.
- Grassi, M., Voinovich, D., Franceschinis, E., Perissutti, B., Filipovic-Grcic, J.Theoretical and experimental study on theophylline release from stearic acid cylindrical delivery systems. Journal of Controlled Release. Volume 92, Issue 3, 30 October 2003, Pages 275-289.
- Passerini, N., Perissutti, B., Moneghini, M., Voinovich, D., Albertini, B., Cavallari, C., Rodriguez, L. Characterization of carbamazepine-Gelucire 50/13 microparticles prepared by a spray-congealing process using ultrasounds. Journal of Pharmaceutical Sciences Volume 91, Issue 3, 2002, Pages 699-707.
- Perissutti, B., Newton, J.M., Podczeck, F., Rubessa, F. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid release dosage form. European Journal of Pharmaceutics and Biopharmaceutics. Volume 53, Issue 1, 2002, Pages 125-132.
- Passerini, N., Albertini, B., Perissutti, B., Rodriguez, L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. International Journal of Pharmaceutics Volume 318, Issue 1-2, 2 August 2006, Pages 92-102.
Research Group
| Solvent-free technological processes Group |