Design, synthesis and biological evaluation of new sigma ligands
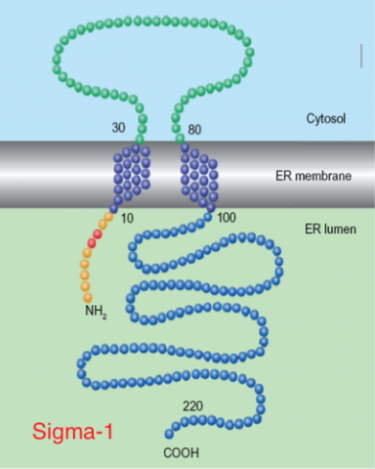
The sigma receptors (σRs) differentiate into two subtypes: σ1 and σ2. Their distribution is mainly in the CNS, but also at a peripheral level and differ in chemical and pharmacological profile, for their molecular weight (MW) and the transduction mechanism. In particular, σ1 subtype has been more extensively studied and its amino acid sequence (223 amino acids and MW= 25 KDa) is known, although it is still unknown its 3D structure. In particular, σ1 receptors are involved at central level in the modulation of the release of various neurotransmitters, such as dopamine and acetylcoline, modulation of the NMDA receptor, the neuro-protective and anti-amnesic activities; σ1 antagonists also induce cytotoxicity in various tumor cell lines. The σ2 receptors, however, have been studied less, and therefore less known; we do not know the amino acid sequence or 3D structure, but only that they have a MW of 18-21 KDa. Their distribution is extended to various levels and, in particular, are overexpressed in various cancer cells in proliferation and quiescent, both of the CNS that at the peripheral level (eg. pancreatic and breast); it was discovered that σ2 agonist ligands induce apoptosis of tumor cells through a caspase-dependent mechanism with a consequent increase in the intracellular Ca2+. On this basis, the research is oriented towards the synthesis of novel, potent and selective σ2 agonists, as potential anticancer. No less interesting is the development of σ1 agonists as possible CNS neuro-protective and possible applications in drug addictions. Our research team takes care of the synthesis of new ligands of both receptor subtypes, and the development of techniques, such as molecular modeling and cellular tests, to increase knowledge of these enigmatic receptors.
Main publications:
1. M.G. Mamolo, D. Zampieri, C. Zanette, C. Florio, S. Collina, M. Urbano, O. Azzolina, L. Vio, “Substituted benzylaminoalkylindoles with preference for the s2 binding site.†Eur. J. Med. Chem. 43 (2008) 2073-2081.
2. D. Zampieri, M.G. Mamolo, E. Laurini, C. Zanette, C. Florio, S. Collina, D. Rossi, O. Azzolina, L. Vio, “Substituted benzo[d]oxazol-2(3H)-one derivatives with preference for the s1 binding site.†Eur. J. Med. Chem. 44 (2009) 124-130.
3. D. Zampieri, M.G. Mamolo, E. Laurini, C. Florio, C. Zanette, M. Fermeglia , P. Posocco, M.S. Paneni, S. Pricl, L. Vio, “Synthesis, biological evaluation, and three-dimensional in silico pharmacophore model for s1 receptor ligand based on a series of benzo[d]oxazol-2(3H)-one derivatives.†J. Med. Chem. 52 (2009) 5380-5393.
4. D.Rossi, M. Urbano, A. Pedrali, M. Serra, D. Zampieri, M.G. Mamolo, C. Laggner , C. Zanette, C. Florio, D. Schepmann, B. Wunsch, O. Azzolina, S. Collina, “Design, synthesis and SAR analysis of novel selective σ1 ligands (Part 2)†Bioorg. Med. Chem., 18 (2010) 1204-1212.
5. E. Laurini, D. Zampieri, M.G. Mamolo, L. Vio, C. Zanette, C. Florio, P. Posocco, M. Fermeglia, S. Pricl, “A 3D-Pharmacophore Model for σ2 Receptors Based on a Series of Substituted Benzo[d]oxazol-2(3H)-one Derivatives†Biorg. Med. Chem. Lett., 20 (2010) 2954-2957.
6. E. Laurini, V. Dal Col, M.G. Mamolo, D. Zampieri, P. Posocco, M. Fermeglia, L. Vio, S. Pricl, “Homology model and docking-based virtual for ligands of the σ1 receptor†ACS Med. Chem. Letters, 2 (2011) 834-839.
7. E. Laurini, D. Marson, V. Dal Col, M. Fermeglia, M.G. Mamolo, D. Zampieri, L. Vio, S. Pricl, “Another brick in the wall. Validation of the sigma1 receptor 3D model by the computer-assisted design, synthesis, activity and activity of new sigma-1 ligandsâ€, Mol. Pharm. 20 (2012) 3107-3126
8. D. Zampieri, E. Laurini, L. Vio, S. Golob, M. Fermeglia, S. Pricl, M.G. Mamolo, “Synthesis and receptor binding studies of some new arylcarboxamide derivative sas sigma-1 ligandsâ€, Bioorg. Med. Chem. Lett. 24 (2014) 1021-1025.
9. D. Zampieri, E. Laurini, L. Vio, M. Fermeglia, S. Pricl, B. Wuensch, D. Schepmann, M.G. Mamolo, “Improving selectivity preserving affinity: new piperidine-4-carboxamide derivatives as effective sigma-1 ligandsâ€, Eur. J. Med. Chem., 90 (2015) 797-808.
Research Group
| Mamolo and Zampieri Group |