Synthesis of enantiomerically pure lactones and evaluation of their biological activity
The lactone ring system is found in a vast array of naturally occurring biologically active compounds. The huge interest in these compounds is related to their broad spectrum biological activities as antimicrobial, anti-tumoral, anti-inflammatory, immunomodulatory, anti-fungal, plant growth regulatory and as key flavours of aged alcoholic beverages. The aim of this project is the synthesis of optically pure lactone derivatives by means of biotransformation reactions.
In particular the project is aimed at:
1) the synthesis and enzymatic resolution of new alfa-methylene-gamma-butyrolactones and derivatives bearing a carboxylic group at C-beta and an alkyl substituent at C-gamma;
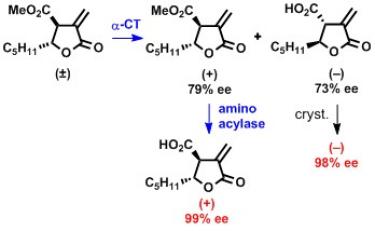
- K. Chakrabarty, I. Defrenza, N. Denora, S. Drioli, C. Forzato, M. Franco, G. Lentini, P. Nitti, G. Pitacco. Enzymatic resolution of α-methyleneparaconic acids and evaluation of their biological activity, Chirality 27, 239–246, 2015
2) the synthesis of enantiomerically enriched macrolides (8 to 11 membered lactones), precursors of hydroxystearic acids, by ring closing metathesis (RCM);
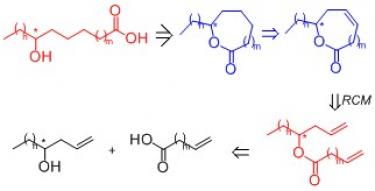
- C. Boga, S. Drioli, C. Forzato, G. Micheletti, P. Nitti, F. Prati. An easy route to enantiomerically pure 7- and 8-hydroxystearic acids by olefin metathesis - based approach. Sent to Synlett, 2015
Research Group
| Chiral lactones Group |