The Department of Chemical and Pharmaceutical Sciences is partner of the EPIC project
While approximately ten percent of cancers originate from genetics, most cancer cases are caused by external factors, such as diet, tobacco, infections, exposure to radiations and chemicals. These external factors can induce epigenetic modifications, i.e., modifications of the chromosomes that do not involve alterations of the DNA sequence.
Epigenetic alterations can occur in genes that encode for tumor suppressors, immunosuppressive cytokines and checkpoint proteins and thus compromise the activation of anti-tumor immunity.
Among the most common epigenetic modifications is the acetylation/deacetylation of histones, proteins associated to DNA in chromatin. Histone acetylation and deacetylation controls gene transcription by modifying the structure of chromatin: acetylation of histones relaxes the chromatin structure, making DNA more accessible to enzymes involved in transcription and eventually activating gene transcription. Deacetylation, on the contrary, compacts the chromatin structure and slows down transcription.
The Epigenetics of Immunity in Cancer (EPIC) project focuses on the inhibition of histone deacetylases (HDACs), i.e., the enzymes responsible for the deacetylation of histones. Inhibition of HDACs keeps histones in the acetylated state and reactivates genes that promote anti-tumor immunity, thereby assisting the immune system in its fight against cancer.
The EPIC consortium started in September 2019 and includes the Universities of Udine (Lead Partner), Trieste, Salzburg, and Eurac Research in Bolzano. Biologists at the University of Udine study the effects of HDAC inhibitors identified from commercial libraries. Scientists at the Paris Lodron University of Salzburg investigate the effects of selected inhibitors on the immune system in cell models and artificial organs. Bioinformaticians at Eurac Research measure differences between cancer cells with and without inhibitors using next generation sequencing and analyze data generated by the consortium. Bioorganic chemists at the University of Trieste design and synthesize new HDAC inhibitors.
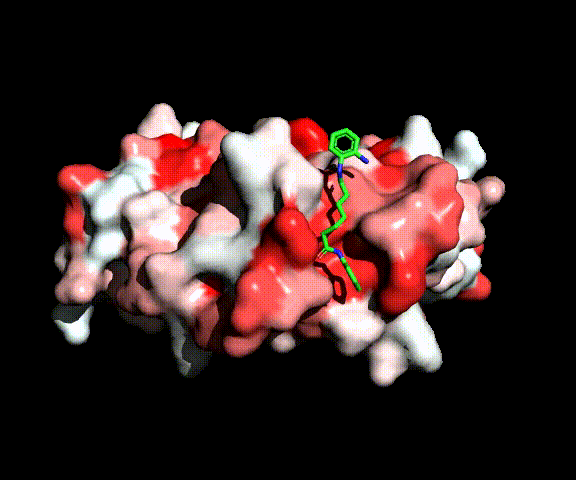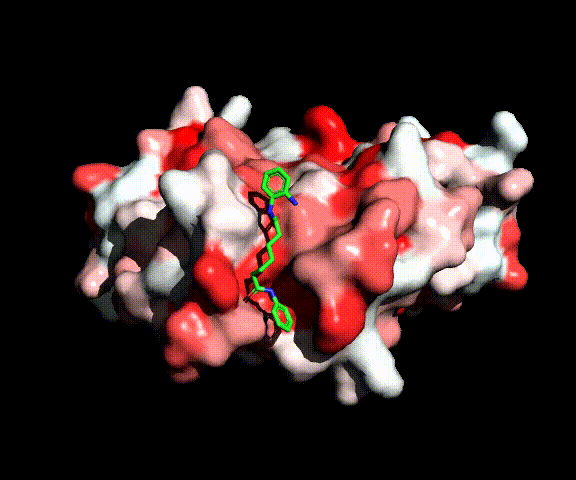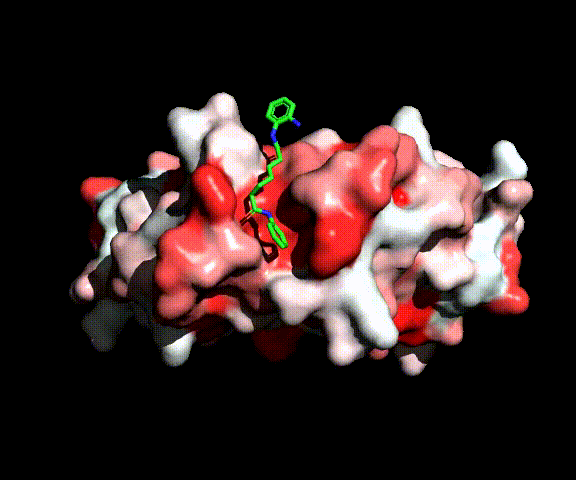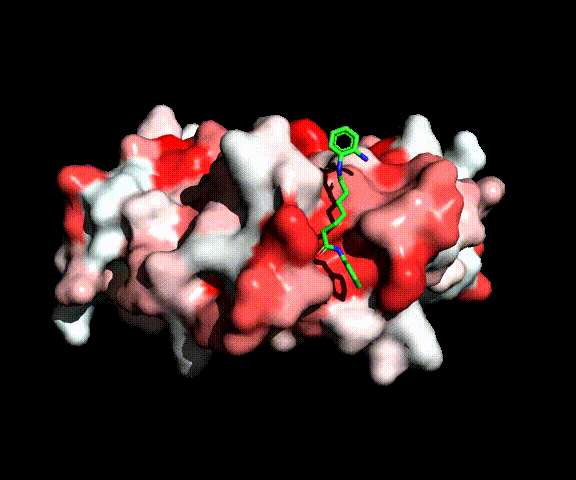
Complex between MEF2 and HDAC inhibitor BML210 (green)
We initially tested the literature hypothesis that diamides of pimelic and suberic acid may inhibit HDAC-2 recruitment by MEF2. We synthesized a series of diamides optimized for binding to MEF2 but lacking the Zn-binding group that is necessary for active-site inhibition of HDACs. None of these compounds had a significative anti-tumor activity, indicating that this mechanism for HDAC inhibition is unlikely. This was confirmed by evaluating the binding energies for the complexes between MEF2 and the new compounds which are one order of magnitude lower than the MEF2-HDAC binding energies. Inhibitory activity was restored by introducing a Zn-binding group indicating that molecules of this class behave as conventional active-site inhibitors when possessing a Zn-binding moiety.
We have then synthesized a series of new inhibitors based on the pimelic diamide structure of inhibitor BML210, in which several different Zn-binding groups were introduced.
The biological activity of the new compounds has been investigated during spring 2022, and actually they resulted active. It is therefore likely that they can interact at the active site level as well as the reference inhibitors HDAC8:
HDAC8 complexed with active site inhibitor SAHA
After this evaluation, and after having obtained this confirmation about the need to put a zing binding group inside the molecules, we have spent the last months of the project designing and synthesizing a final series of derivatives hopefully more active, that retain the ability of interacting at the active site and at the entrance channel, but also capable to occupy the so called nonclassical subsite of such enzymes:
To this end, we have considered a set of dihydropirimidinones (DHMP), starting from our previous experience in the preparation of inhibitors of b-secretase. The novel compounds contain an ureidic group, which can be further functionalyzed with alkohoxyl groups in order to act as zinc finger.
A library of 10 potential inhibitors has been synthesized exploiting the multicomponent Biginelli reaction:
The synthetic activity was continued up to the end of the project, and the compounds have been sent to the lead partner for further measures in the future.
Partners
University of Udine (Lead Partner)
University of Trieste (PP1)
Paris Lodron Universität Salzburg (PP2)
Eurac Research Bolzano (PP3)
Duration
September 2019 - December 2022
Funding
Interreg V-A Italia - Osterreich
Total Budget
€ 940.370,54


