Prodotti Naturali: sintesi, identificazione e valutazione dell'attività biologica; Tecnologie biosensoristiche per analisi agro-alimentari
Natural Products: synthesis, identification and evaluation of the biological activity
Biosensor technologies for agro-food detection
Coffee natural products: chlorogenic acids, hydroxycinnamoyl amides and diterpenes
The coffee plant belongs to the Rubiaceae family, genus Coffea which counts more than 100 different species, but the two commercially exploited species are Coffea arabica, simply known as Arabica, and Coffea canephora, known as Robusta.
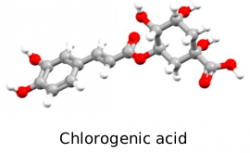
Chlorogenic acids(CGAs) are esters of (-)-quinic acid with different trans-cinnamic acids (such as caffeic, ferulic and p-coumaric acid) and are secondary metabolites belonging to the family of polyphenols which are involved in the defense mechanisms of plants against external agents. CGAs are well known for their antioxidant activities providing positive health effects and, although their concentrations may vary from one source to another, green coffee beans are particularly rich of CGAs, especially of caffeoylquinic acids (CQAs). Their identification is made by means of HPLC/MS analyses but although the HPLC/MS is generally accepted and it is considered a reliable technique, the availability of authentic standards could help in CGAs identification and quantification, without the need of a MS detector but simply using an UV detector. This research group is working on the synthesis of standards of chlorogenic acids and their derivatives which are not commercially available as well as on their biological activity. Part of the research activity of the group in this field is reported in the following publications of this research group:
- V. Sinisi, K. Boronova, S. Colomban, L. Navarini, F. Berti, C. Forzato “Synthesis of Mono-, Di-, and Tri-3,4-dimethoxycinnamoyl-1,5--quinides” Eur. J. Org. Chem. 2014, 1321-1326.
- V. Sinisi, C. Forzato, N. Cefarin, L. Navarini, F. Berti “ Interaction of chlorogenic acids and quinides from coffee with human serum albumin” Food Chemistry2015, 168, 332-340.
- L. Gutierrez Ortiz, F. Berti, L. Navarini, A. Monteiro, M. Resmini, C. Forzato “Synthesis of p-coumaroylquinic acids and analysis of their interconversion” Tetrahedron: Asymmetry2017, 28(3), 419-427.
- V. Sinisi, A. Stevaert, F. Berti, C. Forzato, F. Benedetti, L. Navarini, A. Camps, L. Persoons, K. Vermeire “ Chlorogenic compounds from coffee beans exert activity against respiratory viruses” Planta Medica2017, 83(07), 615-623.
- A. L. Gutierrez Ortiz, F. Berti, W. S. Sanchez, L. Navarini, S. Colomban, P. Crisafulli, C. Forzato “Distribution of p-coumaroylquinic acids in commercial Coffea spp. of different geographical origin and in other wild coffee species” Food Chemistry2019, 286, 459-466.
Hydroxycinnamoyl amides are conjugates of hydroxycinnamic acids with the amino acids phenylalanine, tyrosine, tryptophan or aspartic acid, and although their role in the plant is not known, they exhibit biological activity such as anti-neuroinflammatory activity, hepatoprotective activity, HIV-1 integrase inhibitors, and antidiabetic effects. Moreover, from a sensory point of view, hydroxycinnamoyl amides have been indicated as possible contributor to astringent taste of some food. Hydroxycinnamoyl amides are manly present in the Robusta species, so their identification could contribute in differentiating the two species and also the geographical origin. See for example the recent publication:
- F. Berti, L. Navarini, S. Colomban and C. Forzato “Hydroxycinnamoyl Amino Acids Conjugates: A Chiral Pool to Distinguish Commercially Exploited Coffea spp.” Molecules2020, 25, 1704.
Diterpenes represent the 86–88% of the total unsaponifiable matter for Arabica and approximately 69 % for Robusta, which shows the highest sterolic fraction content.
The free diterpenes cafestol, kahweol and 16-O-methylcafestol (16OMC) are mainly esterified with various fatty acids and in their free form they occour only as minor components in coffee oil. 16OMC is present only in Coffea canephora (Robusta) and therefore it is considered a marker for this species.
Diterpenes have been reported to have both desiderable and udesiderable effects in human health such as cholesterol raising effect, anticarcinogenic activity and anti-inflammatory activity. This research group has studied both the interaction of these diterpenes with serum albumins by means of circular dichroism spectroscopy and fluorescence spectroscopy and their quantification by means of 1HNMR.
- E. Guercia, F. Berti, L. Navarini, N. Demitri, C. Forzato “Isolation and characterization of major diterpenes from C. canephora roasted coffee oil” Tetrahedron: Asymmetry2016, 27, 649-656.
- E. Guercia, C. Forzato, L. Navarini, F. Berti “Interaction of coffee compounds with serum albumins. Part II: diterpenes” Food Chemistry2016, 502-508.
- C. Finotello, C. Forzato, A. Gasparini, S. Mammi, L. Navarini, E. Schievano “NMR quantification of 16-O-methylcafestol and kahweol in Coffea canephora var. robusta beans from different geographical origins” Food Control2017, 75, 62-69.
- F. Berti, L. Navarini, E. Guercia, A. Oreski, A. Gasparini, J. Scoltock, C. Forzato “Interaction of the coffee diterpenes cafestol and 16-O-methyl-cafestol palmitates with serum albumins” Int. J. Molecular Sciences2020, 21, 1823.
Olive oil natural products: tyrosol, hydroxytyrosol, oleuropein and oleocanthal

Olive oil is rich in lipids which are present in 98% in the saponifiable fraction while minor compounds are present in the unsaponifiable fraction. These minor compounds are mainly sterols, fatty alcohols, triterpenes, tocopherols and phenolic compounds.
Among phenolic compounds, the phenolic alcohols tyrosol and hydroxytyrosol, as well as their secoiridoid derivatives oluropein and ligstroside, are considered natural antioxidants.
In 2012 the EFSA approved an health claim on olive oil polyphenols (Commission Regulation (EU) 432/2012 which stats “Olive oil polyphenols contribute to the protection of blood lipids from oxidative stress. The claim may be used only for olive oil, containing at least 5 mg of hydroxytyrosol and its derivatives (e.g. oleuropein complex and tyrosol) per 20 g of olive oil”. Different cultivars are part of the Olea europaea species and different phenolic composition are present which characterize the cultivar. The phenolic composition can change also depending on the geographical origin and agronomical practices as well as climate conditions and it is so important to establish the exact composition of the phenolic compounds to characterize the specific cultivar. In Friuli-Venezia Giulia the cultivar Bianchera/belica is largely present and it is very rich in phenolic compounds contributing in positive nutritional properties of the olive oil derived from it. We are focusing in the complete characterization of the phenolic compounds present in the cultivar Bianchera/belica by means of HPLC-MS technique as well as NMR spectroscopy to recognize its uniqueness.
Synthesis of sensing elements for the development of sensors for natural compounds recognition
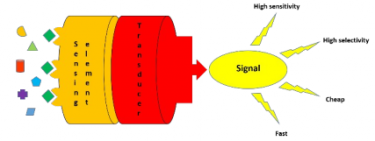
This research group is active in developing sensors for the identification and quantification of the above-cited compounds. The sensing elements considered, which are the core for the development of a self-contained device that is capable of providing real-time analytical information about a test sample, are both small peptides and molecularly imprinted polymers.
In particular, molecularly imprinted polymers are very interesting and versatile recognition elements for sensing and can be immobilized on a support. They are synthetic materials able to specifically and selectively recognize a target compound and are easy to obtain as well as cheap. Moreover, the introduction of a fluorescent group to obtain fluorescent materials ca enhance the sensitivity and the selectivity in order to develop devices for the rapid detection of food quality. The following publications describe this part of the research.
- M. Del Carlo, D. Capoferri, I. Gladich, F. Guida, C. Forzato, L. Navarini, D. Compagnone, A. Laio, F. Berti “In silico design of short peptides as sensing elements for phenolic compounds” ACS Sensors2016, 279-286.
- A. Stavro Santarosa, F. Berti, M. Tommasini, A. Calabretti and C. Forzato “Signal-On Fluorescent Imprinted Nanoparticles for Sensing of Phenols in Aqueous Olive Leaves Extracts” Nanomaterials 2020, 10, 1011.
Gruppo di ricerca
| Gruppo Biosensori |