Catalisi per reazioni di polimerizzazione
Novel catalysts for controlled alkene polymerization and co-polymerization reactions in homogeneous phase
Starting from the discovery by Ziegler and Natta of the first catalyst for controlled alkene polymerization, the development of catalysts for the synthesis of polymeric materials has been an impressively growing research field. Nowadays, it is possible to state that single-site metal catalyzed polymerization represents a powerful tool for the controlled, environmentally friendly, synthesis of new macromolecules. Indeed, thanks to the capability of tailoring the chemical environment on the metal, it is possible to tune the electronic and steric properties of the catalytic centre, allowing the control of the properties of the synthesized macromolecules, i.e. molecular weight and molecular weight distribution, nature of the end groups, stereochemistry, comonomer insertion and its distribution.
The main research interest of Prof. Milani deals with the development of highly efficient homogeneous catalysts for controlled polymerizations. To reach this major objective, the research activity embraces studies on organometallic and coordination chemistry, catalysis, and polymer characterization, mainly addressed to late transition metal complexes with polydentate ligands.
Two main research lines are currently under investigation:
1. Development of catalysts for ethylene/polar vinyl monomer copolymerization;
2. Development of catalysts for CO/vinyl arene copolymerization.
From a general point of view the research activity encompasses of the typical steps of projects in homogeneous catalysis, that are: i. synthesis and characterization of ligands; ii. synthesis and characterization of the related complexes; iii. study of their catalytic behavior in the target reactions; iv. mechanistic investigation; v. characterization of the synthesized macromolecules. In particular, for points i. and v. Prof. Milani takes advantage of the many National and International collaborations established along the years.
1. Development of catalysts for ethylene/polar vinyl monomer copolymerization.
One of the major unsolved problems in the field of polymer chemistry is represented by the synthesis of ethylene/polar vinyl monomer copolymers through the homogeneously catalyzed, direct copolymerization of the two comonomers (Figure 1).
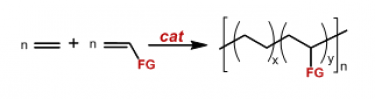
Figure 1. The ethylene/polar vinyl monomer copolymerization.
The aim of this research deals with the development of homogeneous catalysts that are able to efficiently carry out the target reaction under mild conditions of temperature and pressure leading to the copolymer with a content of the polar monomer of at least 20 % and with the polar monomer inserted into the main polymer chain.
The research currently pursued is based on the investigation of Pd-complexes with N-donor ligands, belonging mainly to the family of alpha-diimines, but also of pyridyl-imines.
In particular, for the ethylene/methyl acrylate (MA) copolymerization a new non-symmetric alpha-diimines was introduced, demonstrating that the related catalyst is better performing than those with corresponding symmetric derivatives (Figure 2).1

Figure 2. The new non-symmetric ligand (in red), the corresponding symmetric derivatives (in black) and the related catalyst performance (g P/g Pd = grams of product per gram of palladium).
2. Development of catalysts for CO/vinyl arene copolymerization
Prof. Milani has a consolidate experience in the development of homogeneous catalysts for the controlled synthesis of CO/vinyl arene polyketones through the direct copolymerization of the two comonomers (Figure 3).
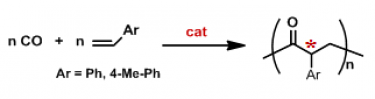
Figure 3. The CO/vinyl arene copolymerization.
In the case of vinyl arenes as alkene comonomers the best performing catalysts are based on palladium complexes with bidentate nitrogen-donor ligands (N-N) and two major issues have been addressed: catalyst stability and polymer stereochemistry.
Two families of precatalysts have been developed, dicationic derivatives of general formula [Pd(N-N)2][X]2, and monocationic species [Pd(CH3)Cl(N-N)][X]. In particular, it was found that an important contribution to the catalyst stability came from the reaction medium, and 2,2,2-trifluorethanol is the solvent choice.2 A strict relationship between ligand symmetry and polymer stereochemistry was established.3
The best performing catalysts for the synthesis of highly syndiotactic copolymer are based on ligands with the 1,10-phenanthroline skeleton: in particular the active species containing the 5,5,6,6-tetrafluoro-5,6-dihydro-1,10-phenanthroline (F4-phen, Figure 4) is active for at least 96 h, leading to the CO/4-methyl styrene polyketone with a Mw = 1000000 (Mw/Mn = 2.3) and with a content of the uu triad of 96 %. The polymer is obtained as a white solid (Figure 4), indicating that no evident decomposition of the catalyst to inactive Pd metal takes place.4


Figure 4. The F4-phen (left) and a sample of CO/4-methyl styrene polyketone (right).
For the synthesis of copolymers with a fully isotactic microstructure, catalysts based on aza-bis-oxazolines (Figure 5) represents a good compromise between productivity, degree of isotacticity and copolymer molecular weight.5

Figure 5. The aza-bis-oxazoline (left) and an example of a non-symmetrically substituted Ar-BIAN (right).
On the other hand, catalysts based on alpha-diimines with an acenaphthene skeleton and with aryl rings symmetrically and non-symmetrically substituted in meta positions (Ar-BIAN, Figure 5) lead to polyketones with an atactic microstructure regardless to the symmetry of the ligand bonded to palladium.6
Finally, we also discovered that when the ligands are substituted in close proximity to the nitrogen-donors the catalytic products are brown oils, CO/vinyl arene cooligomers and not the expected copolymers (Figure 6).7

Figure 6. An example of ortho substituted N-ligand (left) and a sample of CO/ styrene oligoketone (right).
Very recently we have demonstrated that, for the first time, by applying catalysts with a pyrene-tagged pyridyl-immine the stereochemistry of the synthesized CO/vinyl arene polyketones depends on the nature of the vinyl arene leading to syndiotactic copolymer for styrene, to isotactic macromolecules for 4-methyl styrene and to atactic polyketones for 4-tert-butyl styrene.8
Finally, by exploiting the fact that Pd-complexes with alpha-diimines generate catalysts for both the ethylene/MA and the CO/vinyl arene copolymerizations, we have compared the catalytic behavior of complexes with the same ligands in the two reactions. For this studied we choose alpha-diimines with both an acenaphthene (BIAN) and a 1,4-diazabutadiene (DAB) skeleton and substituted with 1-naphthyl or 2-naphthyl groups. We demonstrated that the BIAN-containing catalysts are more suited for the polyketone synthesis, whereas the DAB derivatives generate better performing catalysts for the functionalized polyolefin production.9
References
1. A. Meduri, T. Montini, F. Ragaini, P. Fornasiero, E. Zangrando, B. Milani
Pd-catalyzed ethylene/methyl acrylate cooligomerization: effect of a new nonsymmetric alpha-diimine
ChemCatChem 2013, 5, 1170.
2. B. Milani, A. Anzilutti, L. Vicentini, A. Sessanta o Santi, E. Zangrando, S. Geremia, G. Mestroni
Bischelated palladium(II) complexes with nitrogen-donor chelating ligands are efficient catalyst precursors for the CO/styrene copolymerization reaction.
Organometallics 1997, 16, 5064.
3. G. Consiglio, B. Milani
Stereochemical aspects of co-oligomerization and co-polymerization of alkenes with carbon monoxide.
in “Catalytic synthesis of alkene-carbon monoxide copolymers and cooligomers”
Ed. by Ayusman Sen, Kluwer Academic Press.
2003, Chapter 6; pg. 189.
4. J. Durand,E. Zangrando,M. Stener, G. Fronzoni, C. Carfagna, B. Binotti, P. C. J. Kamer,C. Müller, M. Caporali, P. W. N. M. van Leeuwen, D. Vogt, B. Milani
Long Lived Palladium Catalysts for CO/Vinyl Arene Polyketones Synthesis: A Solution to Deactivation Problems
Chem-Eur. J. 2006, 12, 7639.
5. A. Schätz, A. Scarel, E. Zangrando, L. Mosca, C. Carfagna, A. Gissibl, B. Milani,O. Reiser
High Stereocontrol and Efficiency in CO/Styrene Polyketones Synthesis Promoted by Azabis(oxazoline)-Palladium Complexes
Organometallics 2006, 25, 4065.
6. A. Scarel, M. R. Axet, F. Amoroso, F. Ragaini, C. J. Elsevier, A. Holuigue, C. Carfagna, L. Mosca, B. Milani
Subtle Balance of Steric and Electronic Effects for the Synthesis of Atactic Polyketones Catalyzed by Pd-Complexes with meta-Substituted Aryl-BIAN Ligands
Organometallics 2008, 27, 1486.
7. A. D’Amora, L. Fanfoni, D. Cozzula, N. Guidolin, E. Zangrando, F. Felluga, S. Gladiali, F. Benedetti, B. Milani
Addressing the poly- to oligo-ketone selectivity in styrene carbonylation catalyzed by Pd/bpy complexes. Effect of the 6-alkyl substitution
Organometallics 2010, 29, 4472.
8. G. Canil, V. Rosar, S. Dalla Marta, S. Bronco, F. Fini, C. Carfagna, J. Durand, B. Milani
Unprecedented comonomer dependence of the stereochemistry control in Pd-catalyzed CO/vinyl arene polyketone synthesis
ChemCatChem 2015, 7, 2255.
9. V. Rosar, A. Meduri, T. Montini, F. Fini, C. Carfagna, P. Fornasiero, G. Balducci, E. Zangrando, B. Milani
Analogies and differences in palladium-catayzed CO/styrene and ethylene/methyl acrylate copolymerization reactions
ChemCatChem 2014, 6, 2403
Gruppo di ricerca
| Gruppo Catalisi di polimerizzazione |